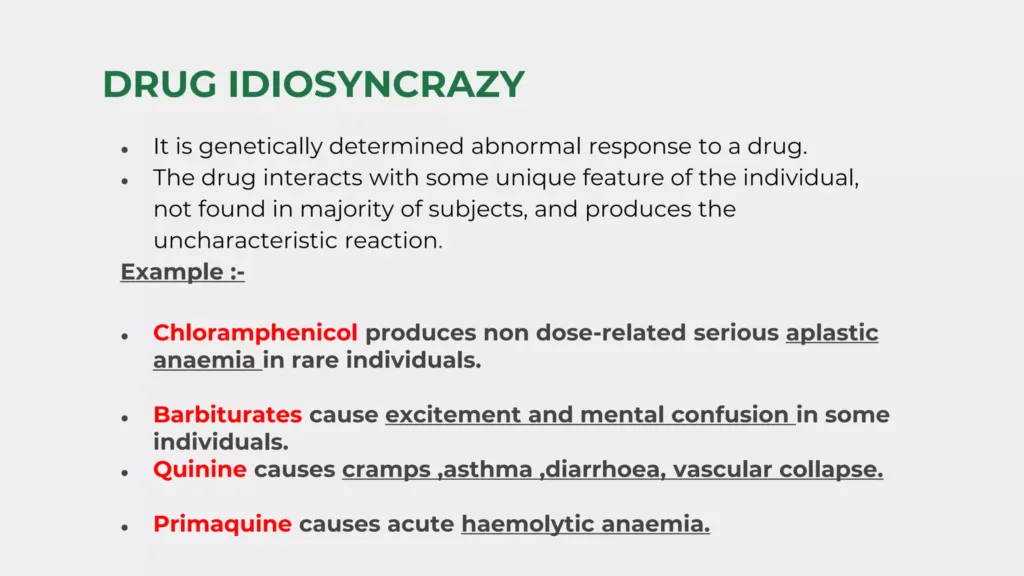
Drug idiosyncrasies (Unforeseen Reactions) are unexpected and abnormal reactions to medications that are not dose-related or related to the known pharmacological properties of the drug. These reactions are typically unpredictable and often result from genetic variations, immune responses, or other individual-specific factors.

Characteristics
- Unpredictability: Reactions cannot be anticipated based on the drug’s usual effects.
- Dose Independence: Can occur at any dose, not necessarily related to high doses.
- Genetic Predisposition: Often linked to genetic differences in drug metabolism or immune system function.
Examples
- Hemolytic Anaemia: In individuals with G6PD deficiency, certain drugs can cause red blood cell destruction.
- Drug-Induced Lupus: Medications like hydralazine and procainamide can trigger lupus-like symptoms in susceptible individuals.
- Steven-Johnson Syndrome (SJS): Severe skin reactions to medications like sulfonamides, anticonvulsants, and NSAIDs.
Contraindications of Crushing Tablets
Crushing tablets can alter the intended absorption, distribution, metabolism, and excretion of the medication, potentially leading to reduced efficacy or increased toxicity. Specific contraindications include:
- Modified-Release Tablets: Crushing can destroy the controlled-release mechanism, leading to rapid drug release and potential overdose.
- Enteric-Coated Tablets: Designed to dissolve in the intestines rather than the stomach, crushing these can cause stomach irritation or reduce the drug’s effectiveness.
- Buccal or Sublingual Tablets: Meant to be absorbed directly into the bloodstream via the mouth lining, crushing these can reduce absorption and efficacy.
- Carcinogenic or Teratogenic Drugs: Crushing these can release harmful dust, posing a risk to those handling the medication.
Conclusion
Understanding drug idiosyncrasies and the contraindications of crushing tablets is crucial for ensuring medication safety and efficacy. Individual patient factors must be considered, and medications should be administered as intended by the manufacturer.
