Preload, afterload, and contractility are essential concepts in cardiovascular physiology that describe different aspects of heart function. Understanding these parameters is crucial for comprehending how the heart pumps blood and how various factors, including medications, can influence its performance.
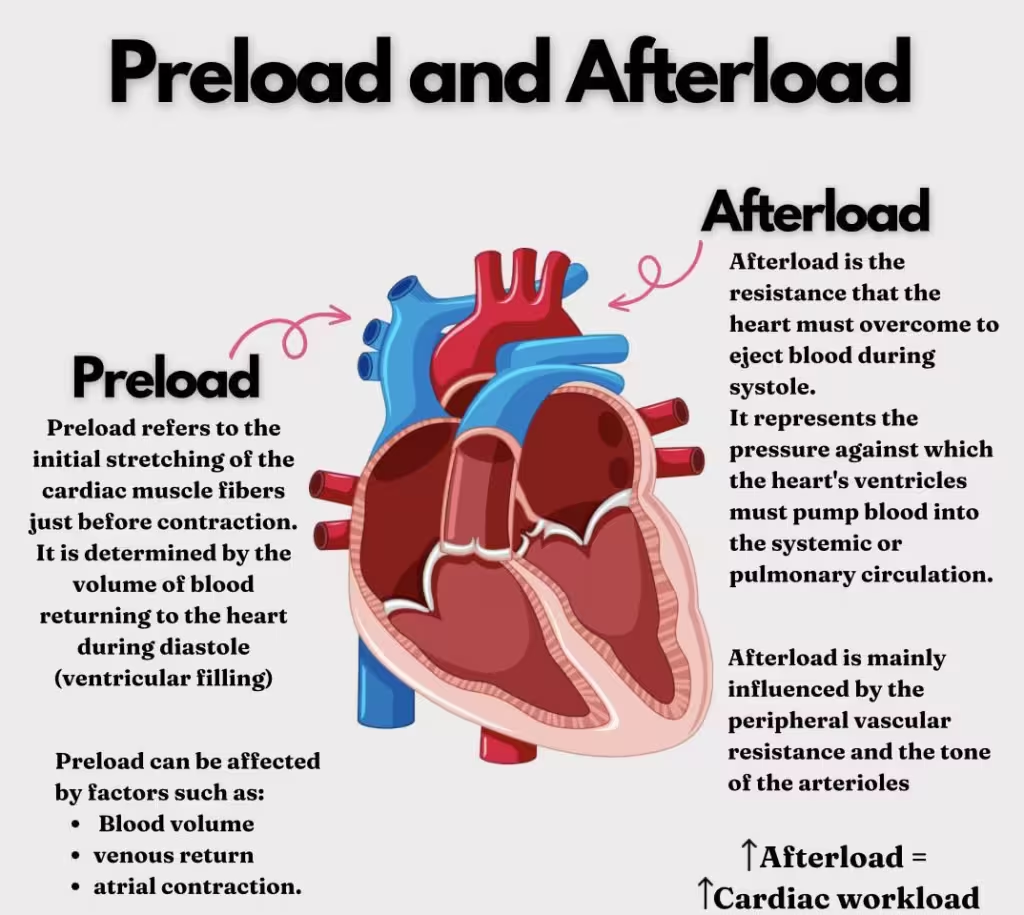
Preload refers to the degree of stretch of the cardiac myocytes (heart muscle cells) at the end of diastole, just before contraction. It is essentially the end-diastolic volume (EDV), which reflects the volume of blood in the ventricles before they contract.
Factors Affecting Preload:
1. Venous Return: The amount of blood returning to the heart. Increased venous return raises EDV, thus increasing preload. Factors such as increased blood volume, muscular activity, and venous tone enhance venous return.
2. Ventricular Compliance: The ability of the ventricles to stretch to accommodate blood. More compliant ventricles can handle larger volumes of blood without significant increases in pressure, thereby increasing preload.
3. Atrial Contraction: Effective atrial contractions contribute to filling the ventricles, enhancing preload.
4. Body Position: Lying down increases venous return compared to standing, thereby increasing preload.
5. Blood Volume: Hypervolemia (increased blood volume) increases preload, while hypovolemia (decreased blood volume) reduces it.
Medicinal Influence on Preload:
1. Diuretics:
– Examples: Furosemide, Hydrochlorothiazide, Spironolactone
– Mechanism: Increase urine output to reduce blood volume, decreasing venous return
and preload.
– Clinical Implications: Used in heart failure and hypertension to prevent fluid overload
and reduce the heart’s workload.
2. Nitrates:
– Examples: Nitroglycerin, Isosorbide dinitrate
– Mechanism: Cause venodilation, reducing venous return to the heart, thereby
decreasing preload.
– Clinical Implications: Treat angina pectoris and heart failure to reduce cardiac workload and oxygen demand.
3. ACE Inhibitors and ARBs:
– Examples: Lisinopril (ACE inhibitor), Losartan (ARB)
– Mechanism: Inhibit the renin-angiotensin-aldosterone system (RAAS), leading to decreased sodium and water retention, which reduces blood volume and preload.
– Clinical Implications: Used in hypertension, heart failure, and chronic kidney disease.
Afterload is the resistance the heart must overcome to eject blood during systole (contraction). It is mainly determined by the pressure in the aorta and the systemic circulation that the left ventricle must overcome to eject blood.
Factors Affecting Afterload:
1. Systemic Vascular Resistance (SVR): Higher SVR increases afterload. SVR can be influenced by arterial tone, affected by vessel diameter, vascular health, and circulating hormones.
2. Aortic Pressure: Increased aortic pressure, as seen in hypertension, raises afterload.
3. Valve Integrity: Conditions like aortic stenosis increase afterload by making it harder for blood to flow out of the left ventricle.
4. Blood Viscosity: Higher blood viscosity increases resistance, thus elevating afterload.
Medicinal Influence on Afterload:
1. ACE Inhibitors and ARBs:
– Mechanism: Block effects of angiotensin II, promoting vasodilation and reducing systemic vascular resistance, thus decreasing afterload.
– Clinical Implications: Effective in lowering blood pressure and reducing heart strain in hypertension and heart failure.
2. Calcium Channel Blockers:
– Examples: Amlodipine, Diltiazem, Verapamil
– Mechanism: Cause vasodilation by inhibiting calcium influx into vascular smooth muscle, reducing afterload.
– Clinical Implications: Manage hypertension, angina, and certain arrhythmias by decreasing vascular resistance and myocardial oxygen demand.
3. Beta-Blockers:
– Examples: Metoprolol, Atenolol, Carvedilol
– Mechanism: Indirectly reduce afterload by decreasing heart rate and contractility, leading to lower cardiac output and reduced blood pressure.
– Clinical Implications: Used for hypertension, heart failure, and ischemic heart disease to reduce myocardial workload and improve survival rates.
4. Direct Vasodilators:
– Examples: Hydralazine, Nitroprusside
– Mechanism: Directly relax vascular smooth muscle, leading to vasodilation and reduced afterload.
– Clinical Implications: Used in hypertensive emergencies and heart failure to rapidly lower blood pressure and decrease cardiac workload.
Contractility
Contractility refers to the intrinsic ability of cardiac muscle fibers to contract at a given preload and afterload, independent of external influences. It measures the strength and efficiency of the heart’s contraction.
Factors Affecting Contractility:
1. Sympathetic Stimulation: Increased sympathetic nervous system activity (via norepinephrine and epinephrine) enhances contractility.
2. Inotropic Agents: Medications like digitalis (Digoxin) and dobutamine can increase contractility.
3. Calcium Levels: Intracellular calcium availability is crucial for muscle contraction. Increased calcium levels enhance contractility.
4. Heart Rate: An increase in heart rate (to a certain extent) can improve contractility through the Bowditch effect (staircase phenomenon), where successive contractions are stronger when the heart beats more frequently.
5. Oxygen Supply: Adequate oxygen supply is necessary for optimal contractile function. Hypoxia or ischaemia can impair contractility.
6. Pathological Conditions: Conditions such as heart failure or cardiomyopathy can decrease contractility.
Medicinal Influence on Contractility:
1. Positive Inotropes:
– Examples: Digoxin, Dobutamine, Milrinone
– Mechanism: Increase intracellular calcium availability or enhance calcium sensitivity in cardiac muscle cells, thereby increasing contractility.
– Clinical Implications: Used in acute heart failure and certain chronic heart failure cases to improve cardiac output and reduce symptoms.
Digoxin has a half life of approximately 36 hours given at average doses in patients with normal renal function.
2. Beta-Agonists:
– Examples: Dobutamine, Dopamine
– Mechanism: Stimulate beta-adrenergic receptors, leading to increased cyclic AMP (cAMP) and enhanced calcium influx, boosting contractility.
– Clinical Implications: Often used in acute heart failure or cardiogenic shock to temporarily support cardiac function.
3. Phosphodiesterase Inhibitors:
– Examples: Milrinone, Inamrinone
– Mechanism: Inhibit the enzyme phosphodiesterase, increasing cAMP levels and enhancing calcium influx, thus increasing contractility.
– Clinical Implications: Utilized in acute heart failure and as a bridge to heart transplantation due to their potent inotropic effects.
Summary
Understanding preload, afterload, and contractility is essential for managing cardiovascular health. Medications play a crucial role in modulating these parameters to treat various heart conditions.
– Diuretics, nitrates, and ACE inhibitors/ARBs primarily reduce preload by decreasing blood volume and venous return.
– ACE inhibitors, ARBs, calcium channel blockers, beta-blockers, and direct vasodilators reduce afterload by promoting vasodilation and lowering vascular resistance.
– Positive inotropes, beta-agonists, and phosphodiesterase inhibitors enhance contractility by increasing calcium availability and sensitivity in cardiac muscle cells.
These mechanisms enable clinicians to tailor treatments that optimize heart function and improve outcomes for patients with heart failure, hypertension, and ischemic heart disease.