Sepsis- (Blood Poisoning) is usually triggered by local infections, such as pneumonia in the lungs, an infected wound or infection of the urinary tract. Bacteria from the local infections spread or secrete toxic substances in to the bloodstream. In response to that the immune system sometimes go into overdrive causing inflammation through the body and you get sepsis.
Definition:
Sepsis is a severe, life-threatening condition that occurs when the body’s response to an infection triggers widespread inflammation, leading to tissue damage, organ failure, and potentially death. Prompt and effective treatment is critical to prevent the progression of sepsis to severe sepsis or septic shock.
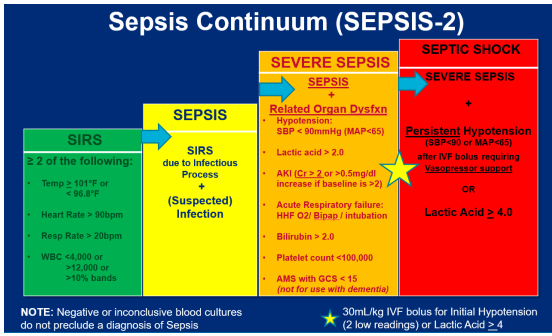
Pathophysiology:
The pathophysiology of sepsis involves a complex interplay of infection, immune response, inflammation, coagulation, and cellular metabolism. Here are the detailed steps:
- Infection and Immune Response:
- Entry of Pathogens: The process begins when pathogens (bacteria, viruses, fungi, or parasites) enter the body through an infection site such as the lungs, urinary tract, abdomen, or bloodstream.
- Recognition: The immune system recognises these pathogens through pattern recognition receptors (PRRs) on immune cells, such as macrophages, dendritic cells, and neutrophils. These receptors identify pathogen-associated molecular patterns (PAMPs) (released by pathogens) and damage-associated molecular patterns (DAMPs)(by Host).
- Cytokine Release: Recognition of pathogens triggers the release of pro-inflammatory cytokines (e.g., TNF-α, IL-1, IL-6). These cytokines recruit more immune cells to the site of infection and initiate the inflammatory response.
2. Systemic Inflammation and Immune Dysregulation: (SIRS)
- Widespread Cytokine Release: The localised infection leads to the systemic release of cytokines into the bloodstream, resulting in a “cytokine storm.” This widespread inflammation causes endothelial cell activation and dysfunction.
- Endothelial Dysfunction: Activated endothelial cells increase the expression of adhesion molecules, promoting leukocyte adhesion and migration into tissues. This leads to increased vascular permeability and leakage of fluid and proteins into tissues, causing oedema and hypovolemia.
- Vasodilation: Inflammatory mediators such as nitric oxide and prostaglandins cause widespread vasodilation, leading to decreased systemic vascular resistance and hypotension. This vasodilation impairs effective tissue perfusion and oxygen delivery to organs.
3. Coagulation Cascade and Microvascular Thrombosis: (DIC)
- Activation of Coagulation: Inflammatory cytokines activate the coagulation cascade, leading to the formation of microthrombi in the small blood vessels. This process is known as disseminated intravascular coagulation (DIC).
- Impaired Fibrinolysis: Inflammatory mediators also inhibit fibrinolysis, the process that breaks down clots, leading to persistent microvascular thrombosis.
- Microvascular Occlusion: Microthrombi occlude capillaries and small vessels, further impairing tissue perfusion and contributing to organ ischemia and dysfunction.
4. Mitochondrial Dysfunction and Cellular Metabolism: (MODS)
- Impaired Oxygen Utilisation: Due to endothelial dysfunction, vasodilation, and microvascular thrombosis, the delivery and utilisation of oxygen by tissues are compromised, leading to cellular hypoxia.
- Mitochondrial Dysfunction: Inflammatory mediators and hypoxia cause mitochondrial dysfunction, reducing ATP production and leading to metabolic failure at the cellular level.
- Cell Death and Organ Dysfunction: Persistent hypoxia, metabolic failure, and the buildup of toxic metabolites result in cellular injury and death. This culminates in the dysfunction of multiple organs, including the lungs (acute respiratory distress syndrome), kidneys (acute kidney injury), liver, and heart.
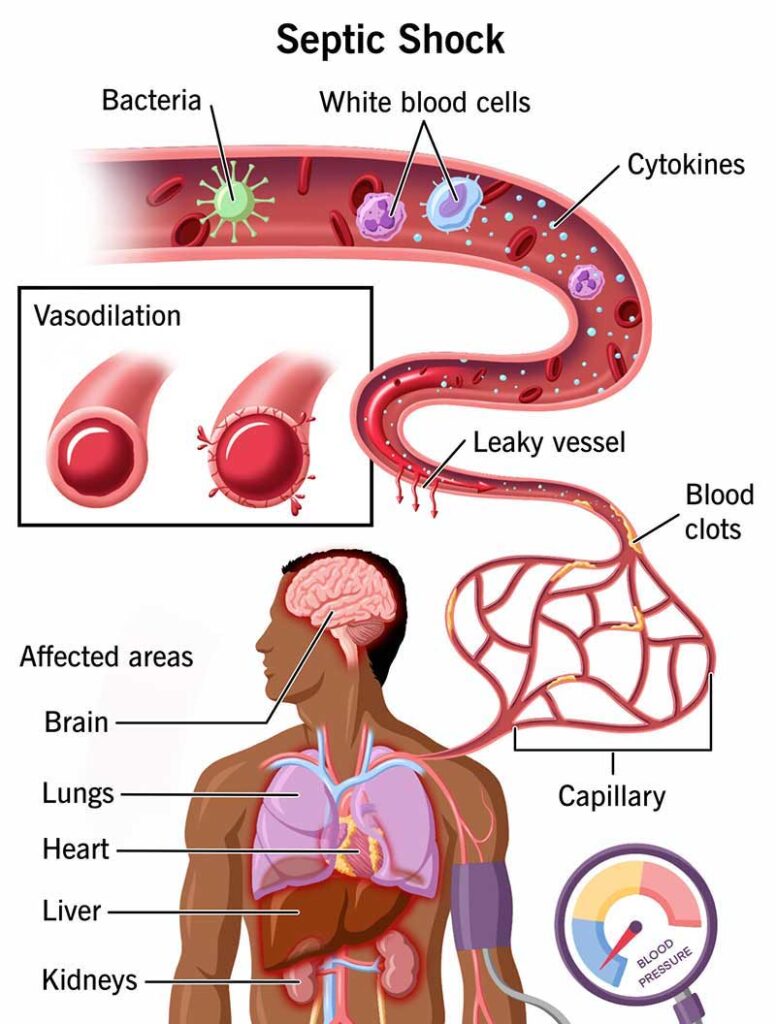
Identification:
Early identification of sepsis is crucial for effective treatment. The following criteria and tools are used to identify sepsis:
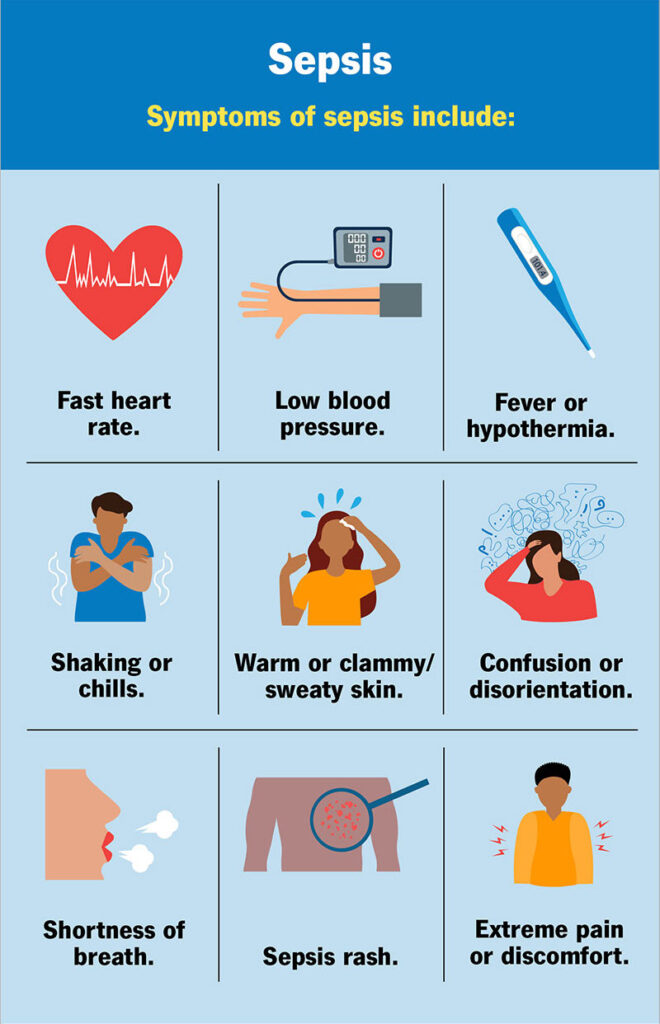
- Clinical Symptoms:
- Fever, chills, or hypothermia
- Tachycardia (rapid heartbeat)
- Tachypnea (rapid breathing)
- Confusion or altered mental state
- Extreme pain or discomfort
- Low blood pressure (hypotension)
- Decreased urine output
2. Laboratory Tests:
- Blood Tests: Elevated white blood cell count (leukocytosis) or low white blood cell count (leukopenia), elevated C-reactive protein (CRP), and elevated procalcitonin levels.
- Lactate Levels: Increased serum lactate levels indicate tissue hypoxia and are used to assess the severity of sepsis.
- Blood Cultures: Positive blood cultures can identify the causative pathogen.
3.. Diagnostic Criteria:
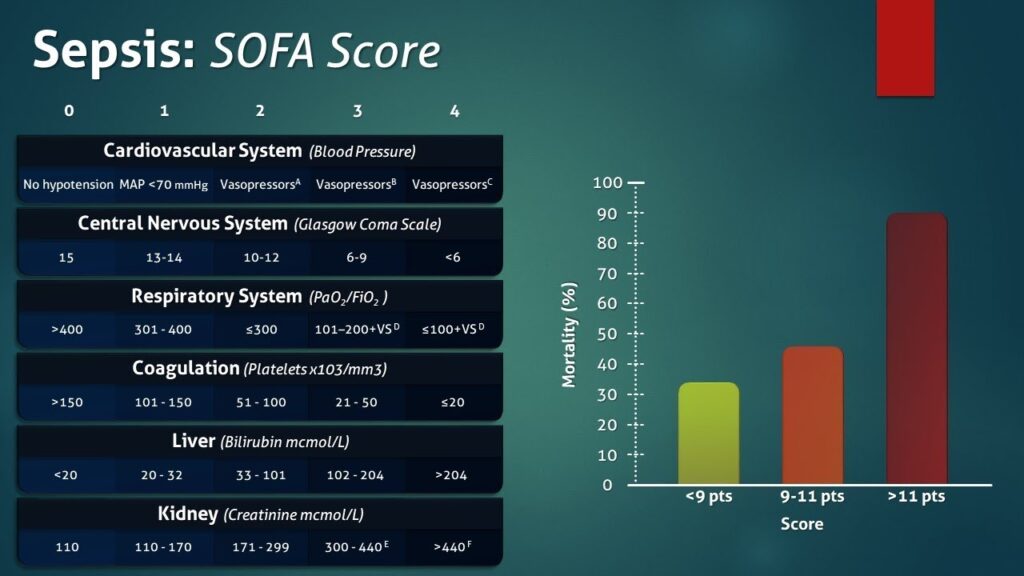
- Sepsis-3 Definition: Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection. Organ dysfunction can be identified by an increase in the Sequential Organ Failure Assessment (SOFA) score of 2 points or more.
- qSOFA Score: Quick SOFA score includes three criteria: altered mental status, systolic blood pressure ≤ 100 mmHg, and respiratory rate ≥ 22 breaths/min. A score of 2 or more suggests a high risk of poor outcomes.
Management:
Effective management of sepsis involves early recognition, rapid administration of antibiotics, and supportive care to stabilise organ function. The following steps are critical: (Kindly Click here for – UK Sepsis Trust Guidelines)
- Initial Resuscitation:
- Fluid Resuscitation: Administer intravenous fluids (crystalloids) to maintain blood pressure and improve tissue perfusion. The goal is to restore effective circulating volume. Administer at least 30 mL/kg of IV crystalloid fluid within the first 3 hours (Surviving Sepsis Campaign Guidelines 2021 )
- Vasopressors: If hypotension persists despite fluid resuscitation, vasopressors ( norepinephrine as the first-line agent ) are used to maintain adequate blood pressure and improve perfusion.
- For adults with septic shock on norepinephrine with inadequate mean arterial pressure levels, we suggest adding vasopressin instead of escalating the dose of norepinephrine (Surviving Sepsis Campaign Guidelines 2021 )

2. Antibiotic Therapy:
- Empiric Antibiotics: Broad-spectrum antibiotics should be administered as soon as possible, ideally within the first hour of recognizing sepsis. The choice of antibiotics depends on the suspected source of infection and local antibiogram data.
- Pathogen-Specific Therapy: Once the causative pathogen is identified through cultures, antibiotic therapy can be adjusted to target the specific organism.
3. Source Control:
- Identify and Eliminate Source of Infection: Drain abscesses, remove infected devices (e.g., catheters), and perform surgery if necessary to control the source of infection.
4. Supportive Care:
- Oxygen Therapy and Mechanical Ventilation: Provide supplemental oxygen or mechanical ventilation to patients with respiratory distress or failure.
- Renal Replacement Therapy: Dialysis may be necessary for patients with acute kidney injury.
- Nutritional Support: Ensure adequate nutritional support for critically ill patients.
5. Monitoring and Follow-Up:
- Frequent Monitoring: Monitor vital signs, urine output, and laboratory parameters regularly to assess response to treatment.
- Adjust Treatment: Continuously reassess and adjust treatment strategies based on the patient’s clinical status and response to therapy.
Early recognition and prompt, aggressive treatment are essential for improving outcomes in patients with sepsis. The management approach must be comprehensive, involving both antimicrobial therapy and supportive measures to address the complex pathophysiological processes involved.
References –
- SCCM | Surviving Sepsis Campaign Guidelines 2021. https://www.sccm.org/Clinical-Resources/Guidelines/Guidelines/Surviving-Sepsis-Guidelines-2021#Recommendations.
